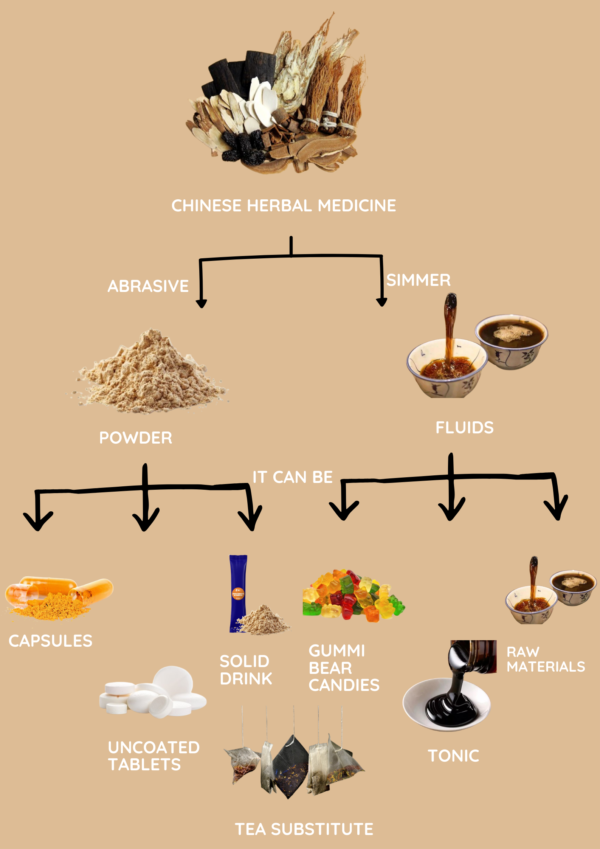
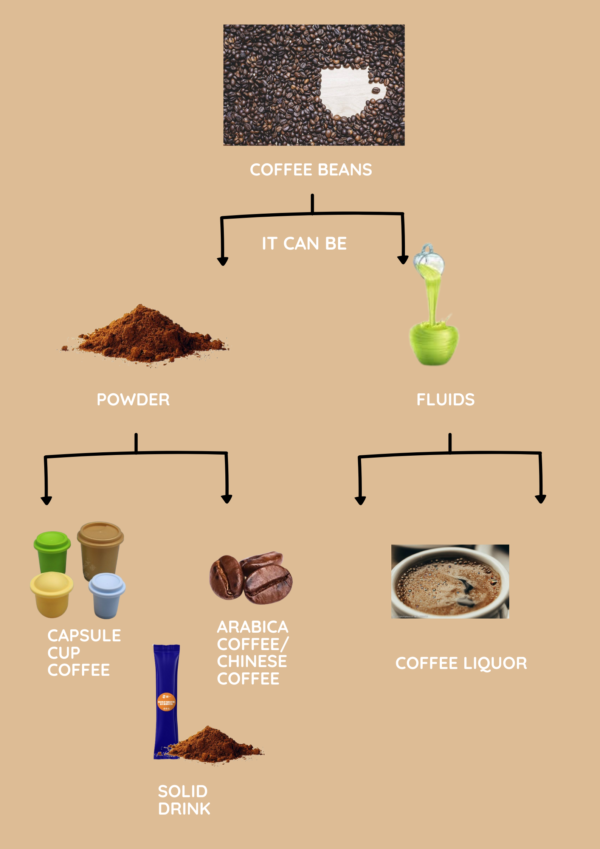
Efficient
Week*6 Day
24-hour start-up
Low cost
1.4 billion people
300 employees
logistic
Delivery to hand
Real-time follow-up

How do we get you to trust us?
As a China-based herbal medicine, health food and dietary supplement manufacturing company specializing in safe, effective and compliant products for brands around the world, all products undergo rigorous third-party testing with certificates of analysis to ensure transparency and quality.
- We will send you a video of each progression to increase trust!
- FDA-registered, cGMP-certified facility for uncompromised quality
- First customized cooperation in small quantities
- Welcome to visit China, we are your best tour guide!
- Available in custom formulations and private label nutraceuticals, we are a trusted partner for brands seeking customized solutions.
OUR SERVICES
We offer services in the manufacturing of

Powder Filling

soft candy

Uncoated Tablets

Capsules

What is GMP registration?
Good Manufacturing Practices (GMPs) are guidelines that provide a system of processes, procedures and documentation to assure a product has the identity, strength, composition, quality and purity that appear on its label. These GMP requirements are listed in Section 8 of NSF/ANSI 173 which is the only American National Standard in the dietary supplement industry developed in accordance with the FDA’s 21 CFR part 111.
NSF International independently registers manufacturers as meeting GMP requirements. The program is open not just to manufacturers of dietary supplements but also to manufacturers of ingredients and raw materials, as well as distribution, warehousing and packaging companies, who want to demonstrate their commitment to public safety.
Independent registration from the global public health organization demonstrates a commitment to dietary supplement quality and compliance with U.S. GMP requirements.
What does it take to be NSF GMP registered?
NSF GMP registration verifies that the facility that is audited twice annually for quality and safety in compliance with Federal Regulations for dietary supplements good manufacturing practices.
Good Manufacturing Practices (GMP) registration including:
- Audits of all production facilities that manufacture, package, warehouse
or distribute dietary supplements or functional foods - Review of quality control testing procedures for raw materials and
finished products. - Review of maintenance, housekeeping, cleaning and sanitization
procedures Review of sourcing and traceability procedures - Review of training and qualification programs Review of internal SOPs
- Product Recall Procedures

What do we do?
Our mission is to provide dietary supplement companies with nutraceutical formulation, highest-quality manufacturing, design & fulfillment services, delivered with unparalleled customer service.
Our Advantages

Dedicated Customer & Account Management
One-on-one with a professional consultant, we will send you a video of every progress! Effective communication at all times

World-Class Turnkey Services
Dosage form, packaging, design, package for your satisfaction

Unmatched Flavoring Capabilites
Work with our in-house Certified Food Scientists to create mouth-watering flavors that your customers will purchase again and again.

Coast-to-Coast Manufacturing Services
Contract manufacturing of dietary supplements in state-of-the-art GMP and ISO certified laboratories. Short production lead time, delivery and after-sales protection.